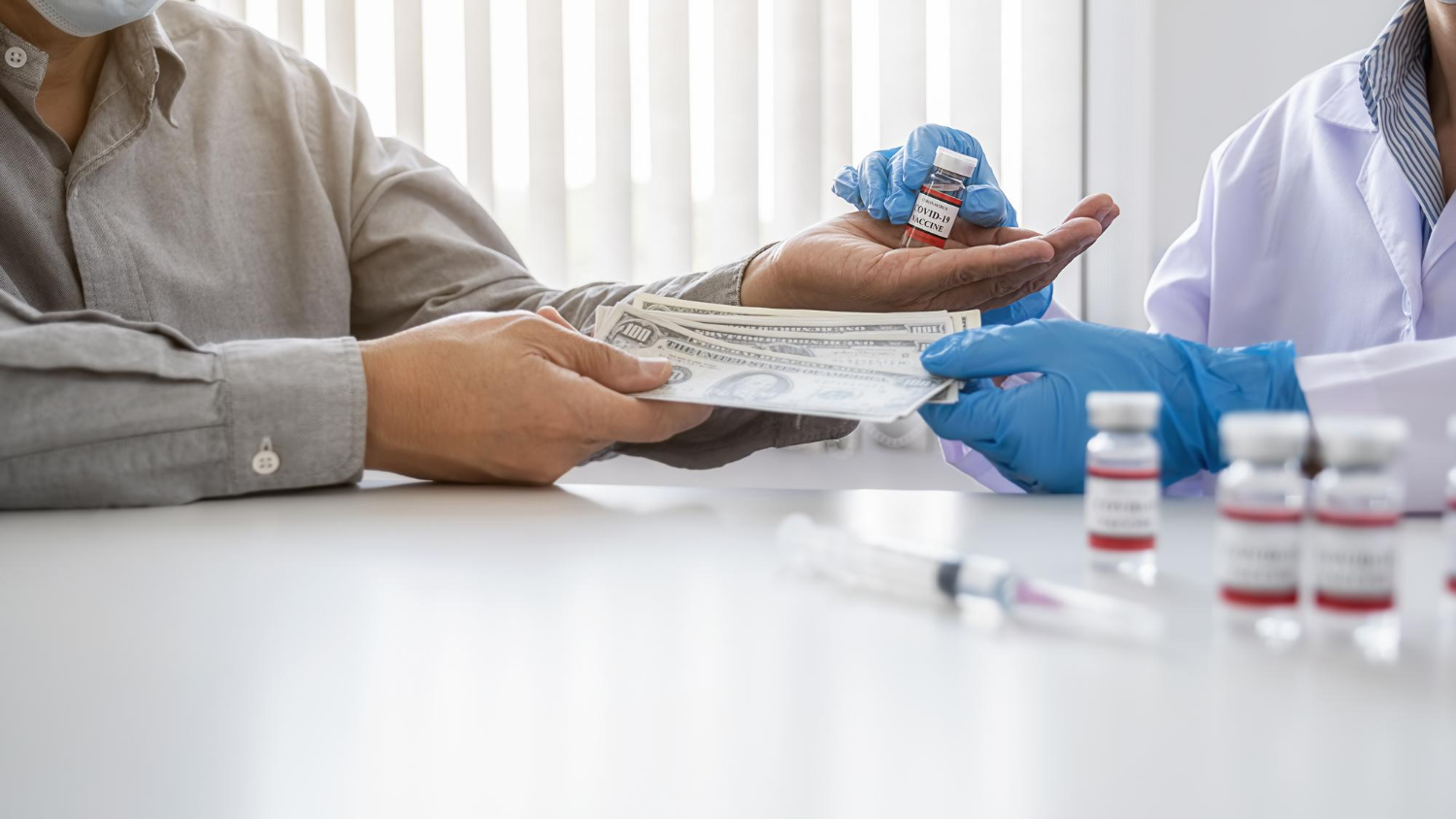
Overview of the Medicare Payment Controversy
A significant battle is unfolding in healthcare policy as manufacturers of expensive wound-care products clash with alliances of hospitals, doctors, and Medicare providers over proposed changes to program spending on advanced bandaging solutions. The controversy centers on “skin substitutes” — high-tech bioengineered products designed to treat complex wounds such as diabetic sores and chronic ulcers.
The Market Size and Scope
The skin substitute industry represents a massive healthcare market segment. According to recent analysis by the National Association of Accountable Care Organizations, this market is projected to exceed $15 billion this year alone. However, this lucrative market has faced persistent scrutiny, with allegations ranging from suspicious billing practices to actual patient harm raising red flags across the healthcare industry.
The Proposed Payment System Changes
Current Medicare Proposal
Medicare administrators are actively evaluating a comprehensive restructuring of how the program reimburses healthcare providers for skin substitutes. Under the proposed system, Medicare would implement a standardized flat fee of approximately $125 per square centimeter for these specialized bandages and grafts.
Projected Financial Impact
The financial implications of this policy shift are substantial. Medicare officials estimate the new payment structure would generate savings of $9.4 billion in the first year of implementation. These projected savings represent a significant reduction in Medicare expenditures, money that could potentially be redirected to other critical healthcare needs or help strengthen the program’s long-term financial sustainability.
Industry Pushback and Lobbying Efforts
Manufacturers’ Opposition
Skin substitute manufacturers have mounted a vigorous resistance campaign against the proposed payment changes. The MASS Coalition, representing industry interests, submitted detailed comment letters to Medicare administrators outlining their concerns. Their position is clear: “Manufacturers will not sell skin substitutes at a loss, and treating providers will not purchase skin substitutes if the reimbursement amounts are insufficient.”
Strategic Lobbying Partnerships
The industry has enlisted powerful political allies in their fight. Ballard Partners, a prominent lobbying firm with established connections to the Trump administration, has been retained to advocate on behalf of wound-care manufacturers. According to Open Secrets data, firms connected to this issue have paid hundreds of thousands of dollars to lobbying organizations this year alone.
Escalating Advocacy Activities
As the decision deadline approaches, wound-care companies have significantly intensified their lobbying presence in Washington. Organogenesis, a major player in the skin substitute market, increased its third-quarter lobbying budget to $300,000 — a remarkable 55% increase compared to the same period last year, according to WP Intelligence tracking data.
Political Connections and Previous Policy Reversals
The Biden-Era Policy Reversal
The current controversy isn’t occurring in a vacuum. Earlier this year, the Trump administration reversed a Biden-era initiative that aimed to control Medicare spending on skin substitutes. This reversal came shortly after a skin substitute manufacturer donated $5 million to the MAGA Inc. PAC, as reported by the New York Times, raising questions about the influence of campaign contributions on healthcare policy decisions.
White House Access
The close relationship between industry leaders and the administration continues. Oliver Burckhardt, CEO of Extremity Care — a skin substitute manufacturer that contributed millions to Trump’s political action committee — attended an exclusive private dinner at the White House last week, demonstrating the level of access these manufacturers enjoy at the highest levels of government.
Healthcare Providers’ Perspective
Accountable Care Organizations Take a Stand
Accountable care organizations (ACOs) — collaborative networks of Medicare providers who receive financial incentives for reducing costs while improving care quality — have emerged as strong supporters of the payment reform. These organizations argue that the proposed flat fee structure is essential to address systemic problems in the current reimbursement system.
Concerns About Current Oversight
ACOs contend that the existing payment framework, which operates with minimal oversight, has created an environment ripe for abuse. They document widespread misuse of skin substitutes and questionable billing practices that inflate costs without delivering proportionate patient benefits.
“Every dollar wasted on ineffective, exploitative care is a dollar diverted from seniors who truly need it — and from strengthening Medicare’s long-term solvency,” stated Accountable for Health on their advocacy website.
Patient Safety Concerns
Documented Cases of Harm
Beyond financial considerations, serious patient safety issues have emerged. Advocacy groups have documented troubling cases where patients received multiple applications of skin substitutes without receiving other essential wound care interventions. In some instances, this incomplete treatment approach resulted in life-threatening infections, raising fundamental questions about how these products are being utilized in clinical practice.
The Stakes for All Parties
For Manufacturers
The proposed payment change represents an existential threat to current business models and profit margins in the skin substitute industry.
For Medicare
The program faces mounting pressure to control costs while ensuring beneficiaries receive appropriate, effective care.
For Patients
Seniors and other Medicare beneficiaries need assurance that they’ll continue to have access to legitimate, beneficial wound-care treatments without being subjected to unnecessary or potentially harmful interventions.
What Comes Next
Decision Timeline
A final determination on the payment structure change is expected by next month, making this a critical period for all stakeholders involved in the debate.
The Power Dynamic
Mara McDermott, CEO of Accountable for Health, offered insight into the political landscape: “I think [CMS officials] are supportive” of implementing the payment system changes. However, she acknowledged the formidable opposition, noting, “The manufacturers have a lot of money to throw at this problem, and they have a lot to lose, and they have a lot of firms, it seems, working on this.”
The outcome of this policy battle will have far-reaching implications for Medicare spending, industry profitability, and most importantly, patient care quality and safety.
Discover the latest GovHealth news updates with a single click. Follow DistilINFO GovHealth and stay ahead with updates. Join our community today!

